The editors and current author would like to thank and acknowledge the significant contribution of the previous author of this chapter from the 2004 first edition Dr. Teresa Bane-Terakubo. This current third edition chapter is a revision and update of the original author’s work.
A 3-year-old female presents to her primary care physician with a chief complaint of an enlarging right-sided neck mass first noted 4 days ago. The mass started as a small lump that has enlarged to the size of a walnut and is now becoming painful with overlying redness. She has had 2 days of fever up to 40 degrees C (104 degrees F). Her appetite for solid foods is decreased, but she is drinking fluids well and her urine output is normal. She has been less active. No one at home has been ill, but she does attend pre-school and several children have been ill recently with sore throats and upper respiratory symptoms. Her history is negative for rhinorrhea, cough, vomiting, diarrhea, recent skin infection, skin rash, weight loss, night sweats, dental problems, exposure to cats or other animals, or travel. Her immunizations are up to date. She is not taking any medications. Her past medical history, family history and social history are noncontributory.
Exam: VS T39, HR 120, RR 20, BP 100/60, oxygen saturation 100% on room air. Weight and height are at the 50th percentile. She appears tired, but is in no distress. Pupils are equal and reactive. Sclera is white and conjunctiva are clear. Tympanic membranes are normal. She has no nasal discharge. Her oropharynx is clear. Her neck is supple with a 3 cm by 4 cm tender, warm, mobile, firm right submandibular mass with overlying erythema; central fluctuance is questionably present. No axillary, supraclavicular, or inguinal lymphadenopathy is noted.
CBC WBC 25.0 with a left shift, CRP is 93 mg/dL. She is admitted to the pediatric ward and started on intravenous clindamycin. An ultrasound of the mass is performed which confirms a moderate central abscess. A surgeon is consulted, and the abscess is incised and drained for a moderate amount of pus under general anesthesia. Gram stain shows numerous white blood cells and gram-positive cocci in clusters. Culture of the pus grows out methicillin-susceptible Staphylococcus aureus. Her antibiotics are changed to intravenous cefazolin. She responds well to the drainage and antibiotics with decreased swelling and pain. She is discharged after 2 days of hospitalization to complete a 10-day course of oral cephalexin.
Lymphadenopathy is a common presenting concern in childhood. Lymphadenopathy (enlargement of a lymph node) is often used interchangeably with lymphadenitis (inflammation of a lymph node). Most children with lymphadenopathy will have a benign, self-limited process. However, some children may have a more serious infection, an underlying serious systemic disease, or malignancy.
Evaluation starts with a thorough history and physical exam. The history should include location (localized versus generalized), duration, associated symptoms (including viral symptoms, sore throat, periodontal disease, skin lesions/infections, arthralgias), recurrent infections, ill contacts, recent travel, exposures (animals, rodents, insects, unpasteurized milk, undercooked meat), sexual activity, immunization status, medications, constitutional or B symptoms (prolonged fever, weight loss, night sweats) suggestive of malignancy. Physical exam of the affected lymph nodes should include location, size, consistency (solid or fluctuant, smooth or nodular, mobile or fixed), presence or absence of tenderness, appearance of the overlying skin (erythematous and warm, violaceous discoloration, drainage tracts). Additional diagnostic clues include signs of infection in areas drained by the affected lymph node(s) (Table 1), conjunctivitis, pharyngitis, periodontal disease, hepatosplenomegaly, bruises, petechiae, and other signs of systemic disease.
Table 1. Body areas drained by some lymph node groups (10)
| Lymph node group | Drainage area |
|---|
| Occipital | Posterior scalp |
| Preauricular | Anterior/temporal scalp, anterior ear canal, pinna, lateral conjunctiva/eyelids |
| Submental | Central lower lip, floor of mouth |
| Submandibular/submaxillary | Forehead, medial eyelids, cheek, nose, lips, posterior mouth submandibular gland |
| Anterior/deep cervical | Head, oropharynx |
| Axillary | Upper arm, anterior/lateral thoracic wall, upper abdominal wall |
| Supraclavicular | Chest, lungs, mediastinum |
| Inguinal | Lower abdomen, genitalia, buttocks, leg |
The history and physical exam can help to identify a benign process, a specific etiology, an increased concern for malignancy, and/or the need for further work-up. Anterior cervical and axillary lymph nodes up to 1 cm, and inguinal lymph nodes up to 1.5 cm, are frequently seen in healthy children and may be observed for resolution. If a specific diagnosis is evident, evaluation and/or treatment can be directed towards that diagnosis; for example, a local infection may be identified in the areas that drain into the affected nodes. Table 1 shows the drainage areas for common lymph node regions. Table 2 summarizes some generalized infections that have predilections for causing lymphadenopathy in certain regions. Signs and symptoms concerning for malignancy warrant more urgent investigation. These symptoms include: a supraclavicular node, nodes 2 cm or larger in children, 1cm or larger in neonates, rapidly growing nodes, matted, fixed, nontender nodes, hepatosplenomegaly, systemic symptoms or the so-called B symptoms (fever for more than 1 week, night sweats or weight loss more than 10% body weight). If no worrisome symptoms are noted and the etiology is still unclear, then the approach can be more stepwise and customized based on duration of symptoms and location of lymph nodes.
Table 2. Lymph node groups affected and commonly implicated infectious diseases.
| Lymph node group | Disease |
|---|
| Preauricular | Cat scratch disease, tularemia*, adenovirus |
| Submandibular, anterior/deep cervical | Viral respiratory infections, EBV, CMV, HIV, Cat scratch disease, mycobacteria, toxoplasmosis, tularemia*, brucellosis*, monkeypox* |
| Posterior cervical | EBV, mycobacteria, toxoplasmosis, rubella |
| Axillary | Cat scratch disease, toxoplasmosis, tularemia*, Yersinia pestis*, monkeypox* |
| Inguinal | Sexually transmitted infections, cat scratch disease, tularemia*, Yersinia pestis*, filariasis* |
*Uncommon in Hawai‘i
CMV=cytomegalovirus, EBV=Epstein-Barr virus, HIV=human immunodeficiency virus
The duration of lymphadenopathy can be divided into 3 categories: acute (less than 2 weeks) versus subacute (2 to 6 weeks) versus chronic (more than 6 weeks). Table 3 shows the common causes of cervical lymphadenopathy classified by duration. Acute lymphadenopathy is further classified based on location and whether lymphadenopathy is localized or generalized (involving two or more noncontiguous lymph node regions, including bilateral). See Figure 1.
Table 3. Cervical lymphadenopathy classified by their duration and common associated pathologies.
| Duration | Diagnoses |
|---|
| Acute (<2 weeks) | Reactive lymphadenopathy (common respiratory viruses, EBV, CMV, HIV, mmps, monkeypox*), bacterial (Staphylococcus aureus, GAS, anaerobes, gram-negative bacilli, toxoplasmosis, leptospirosis, rat bite fever, tularemia*, brucellosis*, Yersinia pestis*), fungal (histoplasmosis, coccidiomycosis, blastomycosis)*, dental infection, Kawasaki disease, MIS-C |
| Subacute (2 to 6 weeks) | Cat scratch disease, NTM, toxoplasmosis, TB, EBV, CMV, HIV, Monkeypox* |
| Chronic (>6 weeks) | Lingering subacute etiologies, autoimmune SLE, JIA, sarcoidosis, serum sickness generalized, Kikuchi-Fujimoto disease, Castleman disease, Rosai-Dorfman disease, Kimura disease, PFAPA syndrome (recurring), medications, storage disease, oncologic, lymphoma, leukemia, solid tumor/metastatic, Langerhans cell histiocytosis, HLH |
*Uncommon in Hawai‘i
CMV=cytomegalovirus, EBV=Epstein-Barr virus, GAS=group A Streptococcus, HIV=human immunodeficiency virus, HLH= hemophagocytic lymphohistiocytosis, JIA=juvenile idiopathic arthritis, MIS-C=Multisystem Inflammatory Syndrome in Children, NTM=nontuberculous mycobacteria, PFAPA=periodic fever, aphthous ulcers, pharyngitis, cervical adenitis, SLE=systemic lupus erythematosus, TB=tuberculosis
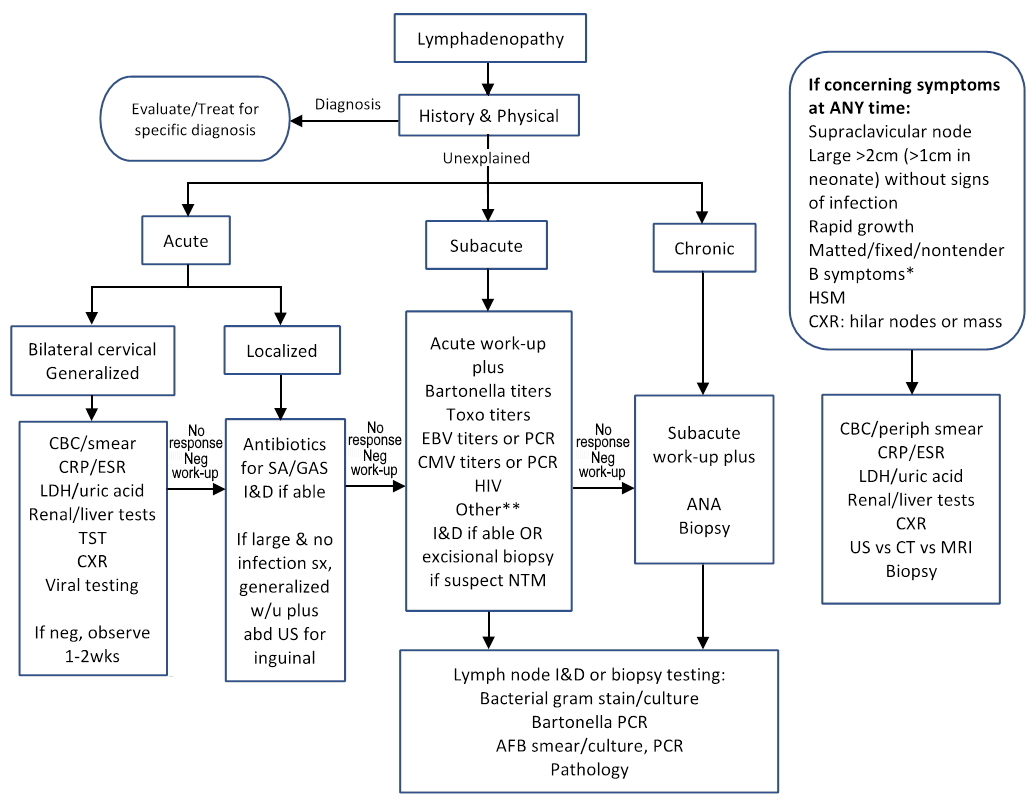
Figure 1. Approach to management of pediatric lymphadenopathy. Adapted from (10,17)
Acute (< 2 weeks), subacute (2 to 6 weeks), chronic (> 6 weeks)
*B symptoms = fever >1week, night sweats, weight loss >10%
**Workup for uncommon diseases according to geographical, or other specific exposures or risk factors
AFB=acid fast bacilli, ANA=antinuclear antigen, Bartonella=Bartonella henselae, CRP=C-reactive protein, CT=computed tomography, CXR=chest radiograph, ESR=erythrocyte sedimentation rate, GAS=group A Streptococcus, HIV=HIV Antigen/Antibody COMBO, HSM= hepatosplenomegaly, I&D=incision and drainage, LDH=lactate dehydrogenase, MRI=magnetic resonance imaging, PCR=polymerase chain reaction, SA=Staphylococcus aureus, sx=symptom, Toxo=Toxoplasma gondii, TST=tuberculin skin test, US=ultrasound
One common presentation is that of acute, bilateral, cervical lymphadenopathy. This usually represents reactive, self-limited lymphadenopathy most commonly due to a viral infection. Viruses commonly implicated include respiratory viruses (e.g., rhinovirus, influenza, and respiratory syncytial virus) and viruses that, while causing systemic inflammation, often include cervical lymphadenopathy such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV). Associated symptoms, epidemiology, history of exposure or clinical findings may help narrow the differential. An additional etiology that must be considered, in the right circumstances, is human immunodeficiency virus (HIV), which during the acute infection can present with fever, sore throat and cervical lymphadenopathy (the acute retroviral syndrome).
Another common presentation is that of acute, unilateral, lymphadenopathy; this is most commonly due to bacterial infections, usually Staphylococcus aureus and group A streptococcus (Streptococcus pyogenes). Staphylococcus aureus and group B streptococcus are the most common pathogens in neonates with acute cervical lymphadenitis (1). In older children, Staphylococcus aureus is particularly common when a node is suppurative. Methicillin resistant Staphylococcus aureus (MRSA) remains common, despite some decrease in its prevalence following its peak in the early 2000s; however, the prevalence is highly variable according to geographic locale. Bacteria from the oral flora including anaerobes and gram-negative bacilli are seen in a small percentage of cases, and particularly when there is associated periodontal disease. In cases of unilateral lymphadenopathy with no signs of acute pyogenic infection (such as erythema, warmth, or tenderness), additional etiologies should be considered, such as Bartonella henselae (cat scratch disease), mycobacteria and malignancy.
Cat scratch disease is one of the most common causes of subacute and chronic localized lymphadenopathy in children. It is caused by the pleomorphic gram-negative bacillus Bartonella henselae, transmitted from scratches or bites of cats, especially kittens, though also detected in fleas and dogs (10). Axillary or epitrochlear nodes are most commonly affected, followed by cervical nodes. Cat scratch disease is the most common cause of Parinaud’s oculoglandular syndrome, even though this syndrome is seen in only 5% to 7% of cases. Affected lymph nodes typically have less signs of inflammation compared to typical acute bacterial infections. Most cases are mild and self-limited, but severe disseminated disease can rarely occur (1,11).
Nontuberculous mycobacteria (NTM) and Mycobacteria tuberculosis (MTB) cervical lymphadenitis are compared in Table 4. Lymphadenopathy is typically painless, and the overlying skin may become violaceus. In contrast to tuberculous, nontuberculous mycobacteria affect younger children, more commonly presents with unilateral cervical lymphadenitis, is more likely to lead to sinus tract formation, is more frequently associated with a normal chest radiograph, a weakly positive tuberculin skin test, and a negative interferon-gamma release assay (IGRA) such as QuantiFERON-TB Gold or T-SPOT; however, it should be noted that a few NTM species may also produce a positive IGRA.
Table 4. Differential characteristics of nontuberculous mycobacteria (NTM) vs Mycobacterium tuberculosis (MTB) cervical lymphadenitis (1).
| NTM | MTB |
|---|
| Age (typical) | 1 to 6 years old | >4 years old |
|---|
| Abnormal CXR | Uncommon | Often |
|---|
| TST >15mm | Uncommon | Often |
|---|
| Positive IGRA | No* | Yes |
|---|
| Bilateral cervical LAN | Uncommon | Not uncommon |
|---|
*Except for infection with M. kansasii, M. szulgai, M. marinum, M. flavescens
CXR=chest radiograph, IGRA=interferon-gamma release assay, LAN=lymphadenopathy, TST=tuberculin skin test
Chronic, recurrent cervical lymphadenitis is seen in the syndrome known as periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA). Periodic fever is defined as temperature greater than 39 degrees C (102.2 degrees F) for 3 to 7 days occurring every 2 to 8 weeks for at least 6 episodes. Pharyngitis, or both aphthous stomatitis and cervical adenopathy, should be present. Additional symptoms may include abdominal pain, diarrhea, or headache. Onset is typically in children under 5 years of age, with normal growth and development and no underlying autoimmune or autoinflammatory disease, immunodeficiency, malignancy, or infection. Symptoms are responsive to corticosteroids (1,16).
Toxoplasmosis presents with acute or subacute painless, non-suppurative, localized lymphadenopathy; most commonly cervical, but occasionally generalized. It is caused by the protozoan Toxoplasma gondii, and organisms are commonly passed in cat feces. Risk factors include exposure to cat feces (litter box), raw pork, lamb, or venison (intermediate hosts), unpasteurized milk, unwashed fruits, or vegetables. Acute symptoms are flu-like, and chronic symptoms include rash, hepatitis, encephalitis, or myocarditis (2).
Leptospirosis is a zoonotic infection that can present with acute generalized lymphadenopathy and systemic symptoms. Systemic symptoms range from mild to severe, with headache, aseptic meningitis, abdominal pain, myalgia, conjunctivitis, rash, jaundice, liver failure, renal failure, pulmonary hemorrhage, and cardiac arrhythmias. It is typically acquired through a skin abrasion or mucous membrane (conjunctiva) that comes in contact with fresh water contaminated with urine or tissue from rodents, livestock (cattle, pigs), or domestic animals. It is more common in tropical climates with Hawai’i having the highest incidence in the United States (3).
Tularemia, brucellosis, plague (Yersinia pestis), and fungal diseases are relatively uncommon but, when present, they frequently associate with acute lymphadenopathy. Tularemia is a zoonotic disease transmitted from rabbits, rodents, and ticks. The most common presentation is that of acute ulceroglandular disease, with a focal skin ulcer at the site of the tick bite and painful regional lymphadenopathy. Less common is oculoglandular disease, associated with preauricular lymphadenopathy. Tularemia has been reported in all states in the United States except Hawai’i. Brucellosis is a zoonotic disease of cattle, sheep, goats, and pigs, associated with ingestion of unpasteurized milk, cheese, or raw meat. It presents with acute or subacute generalized lymphadenopathy in 10% to 20% of cases, and associated flu-like symptoms. It is rare in the US, including Hawai’i. Yersinia pestis causes bubonic plague. The disease is transmitted by an infected flea and presents with acute painful lymphadenopathy (buboes), most commonly inguinal. Sporadic cases occur in the southwestern US, but there has not been any local transmission in Hawai’i since 1949 (4). Coccidiomycosis, histoplasmosis, and blastomycosis are fungal diseases that can present with lymphadenopathy. Coccidiomycosis is endemic to the southwestern US. Histoplasmosis and blastomycosis infections occur in the midwestern, central and eastern US with histoplasmosis spreading more west to Texas (5).
Monkeypox is a zoonosis caused by the monkeypox virus, endemic to West and Central Africa. While rare in the US, there have been outbreaks in 2003 and in 2022 as part of a multi-country outbreak that has included cases in Hawai’i. It presents similarly to smallpox, except that lymphadenopathy is a distinguishing feature. Spread is through close contact typically with an infected rodent, but person-to-person transmission can occur through lesions, body fluids, fomites and respiratory droplets. A prodrome occurs within 1 to 2 weeks of exposure, characterized by nonspecific symptoms of fever, headache, weakness, myalgia, and localized or generalized, acute and subacute lymphadenopathy. A rash follows that progresses from macules to well-circumscribed, often centrally umbilicated, vesicles or pustules, then scabs with all lesions simultaneously progressing through the same stage of development over 2 to 3 weeks. The rash is typically centrifugal, but in the present 2022 outbreak, lesions are localized to the anogenital region. Infectivity ends when the scabs have fallen off. Case fatality for the less severe West African type of monkeypox is 1% and up to 11% for the more severe Central African type. Pre and post exposure smallpox vaccination can prevent or reduce symptoms and antivirals used for smallpox (e.g., tecovirimat) and vaccinia immune globulin, may be beneficial for populations at high risk for severe disease. Vaccination and antivirals are available from the US Strategic National Stockpile (6,7,8).
Kawasaki disease and Multisystem Inflammatory Syndrome in Children (MIS-C) may present with acute cervical lymphadenopathy, unilateral more commonly than bilateral. Kawasaki disease presentation includes fever for 5 or more days, conjunctivitis, rash, erythematous mucous membranes, and erythema and/or edema of the hands and feet. While part of the syndrome, lymphadenopathy is the least common of the findings in Kawasaki. The etiology of Kawasaki is unknown and is likely multifactorial. MIS-C follows 2 to 6 weeks after SARS-CoV-2 infection. Although MIS-C shares similar features to Kawasaki disease, the case definition includes fever, laboratory evidence of inflammation, involvement of 2 or more organ systems (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, neurologic) and evidence of SARS-CoV-2 infection or exposure within 4 weeks; the definition does not include lymphadenopathy, but it is frequently detected (9). Similar to Kawasaki disease, coronary artery dilation is seen in MIS-C. Cardiac dysfunction is frequently more severe in MIS-C than Kawasaki. Children with MIS-C tend to be older than those with Kawasaki disease.
Unlike acute lymphadenopathy, chronic lymphadenopathy is less likely to be infectious, and the diagnosis frequently requires a biopsy. The differential diagnosis is depicted in Table 3 and some examples are highlighted in the following paragraphs.
Kikuchi-Fujimoto disease causes benign, self-limited lymphadenopathy, usually unilateral, painful, posterior cervical, that typically lasts 3 to 6 months. Lymphadenopathy can also be generalized. Asian females are most commonly affected. The etiology is unknown, but infectious and autoimmune triggers are suspected. Systemic symptoms may be seen including fever, malaise, headache, vomiting, sore throat, arthritis, weight loss, night sweats, and nonspecific rash. Laboratory testing is usually normal, but may include leukopenia, anemia, thrombocytopenia, mildly elevated erythrocyte sedimentation rate, transaminitis, positive antinuclear antibody, or increased lactate dehydrogenase. A biopsy is required to establish the diagnosis. The course is usually self-limited, and the treatment is symptomatic. Corticosteroids may shorten the duration of disease in more severe or persistent cases. Symptoms may recur in 3% to 4% of cases. Autoimmune diseases, most commonly systemic lupus erythematosus, may be diagnosed either preceding, concomitant with, or postdating Kikuchi-Fujimoto disease (1,12).
Castleman disease is a rare, benign lymphoproliferative disorder seen in less than 1 in 100,000 population; only a small percentage of which occur in children. Localized disease (unicentric) is significantly more common than disseminated (multicentric) disease and cervical lymphadenopathy is more common in children than adults. Elevated IL-6 is seen. Treatment for localized disease is excision. Treatment for disseminated disease includes chemotherapy, radiotherapy, and IL-6 targeted agents (13).
Rosai-Dorfman disease, also called sinus histiocytosis with massive lymphadenopathy or non-Langerhans cell histiocytosis may be associated with neoplasms and autoimmune diseases. The most frequent presentation is massive, bilateral painless cervical lymphadenopathy. Inguinal, retroperitoneal, mediastinal adenopathy may be seen, and extranodal disease (lytic bone lesions, central nervous system lesions) may be seen in over 40% of cases. Systemic symptoms include fever, weight loss, night sweats. The etiology is unknown but altered immune response or infectious triggers are suspected. The diagnosis is based on histopathology with non-Langerhans sinus histiocytes identified on lymph node biopsy (14).
Kimura disease is a rare, typically benign and self-limited, chronic inflammatory disease seen in young Asian males, characterized by painless, unilateral, cervical adenopathy, or head or neck subcutaneous masses, eosinophilia and elevated serum IgE level. The etiology is unknown but an infectious, allergic, or autoimmune trigger is suspected. Renal disease may be seen. Recurrence is common and treatment may be difficult, involving a multitude of interventions including excision, radiation, corticosteroids, and cytotoxic agents (15).
Chronic generalized lymphadenopathy may be caused by an autoimmune disease (systemic lupus erythematosus, juvenile idiopathic arthritis, sarcoidosis), storage diseases, medications, or oncologic disease.
In most cases, history and physical exam suggest a minor infection and most children do not require much, if any, work-up. Figure 1 summarizes the work-up to consider if the history and physical do not identify a specific diagnosis. Acute localized lymphadenitis with signs suggestive of bacterial infection (erythema, tenderness, warmth, fluctuance) typically does not require additional work-up and is empirically treated with antibiotics and incision and drainage, if applicable. An ultrasound of the lesion can more accurately determine if an abscess is present or if the lymphadenopathy is solid to better determine the need for incision and drainage. If the localized lymph node is large or lacks signs of bacterial infection, it may be prudent to send screening tests (see Figure 1) while empirically treating for the common bacterial infections. Presentation of acute, bilateral cervical lymphadenopathy without signs of a respiratory viral infection, or generalized lymphadenopathy, may also represent more severe disease so laboratory tests screening for systemic disease may be helpful even if early in the course. Lymphadenopathy that persists more than 2 weeks warrants screening laboratory testing for systemic disease, additional testing for cat scratch, mycobacteria, toxoplasmosis, Epstein-Barr virus (EBV), cytomegalovirus (CMV), human immunodeficiency virus (HIV) and consideration for additional testing based on geographical and exposure risk factors.
Comprehensive work-up, including biopsy, should be performed anytime there are concerning findings suggestive of malignancy. These findings may include: supraclavicular node(s), large nodes (more than 2 cm in a child or more than 1cm in a neonate), rapidly growing nodes, fever lasting more than 1 week, night sweats, weight loss more than 10% of body weight, matted, fixed, or nontender nodes, hepatosplenomegaly, chest radiograph with hilar adenopathy or mediastinal mass. Biopsy should also be considered if the diagnosis is uncertain after 3 to 4 weeks of evaluation and/or treatment (17). Excisional biopsy is both diagnostic and therapeutic for nontuberculous mycobacteria and has been associated with better outcomes than antibiotics alone or incisional biopsy which can lead to chronically draining sinus tracts and possibly a higher rate of facial nerve palsy (18). Excisional biopsy is also preferred over fine needle aspiration for ruling out malignancies.
Lymph node ultrasound may be helpful if fluctuance is not obvious on exam. Abdominal ultrasonography may be helpful for unexplained inguinal lymphadenopathy. Additional imaging can be diagnostic, but one study suggested that imaging within 24 hours of presentation correlated with a greater likelihood of multiple imaging studies and a longer length of stay (19). This could be interpreted that early imaging causes these consequences, but there are other interpretations since more complex cases that are more serious and difficult to diagnose are more likely to utilize imaging. Concerns in the use of computed tomography scan include radiation exposure and iodinated contrast risk that has been associated with decreased thyroid hormone levels in children less than 3 years of age (20).
Most cases of acute bilateral cervical lymphadenopathy are viral in etiology, self-limited, and therefore do not require treatment.
For children with acute unilateral suppurative lymphadenitis, antibiotic therapy with coverage for Staphylococcus aureus and group A Streptococcus is recommended. Coverage for methicillin resistant Staphylococcus aureus (MRSA) may be necessary if the local prevalence is high. Clindamycin resistance of both Staphylococcus aureus and group A Streptococcus can be high as well (21). Trimethoprim/sulfamethoxazole and doxycycline while typically effective for MRSA, are less effective against group A Streptococcus. Small abscesses may respond to antibiotics alone while larger abscesses require fine needle aspiration or drainage (22). Ill-appearing children, those with a large area of infection or those who fail outpatient therapy require hospital admission for intravenous antibiotics. With appropriate treatment, clinical improvement should be expected within 48 hours.
Additional considerations for treatment are tailored according to the specific diagnoses. Treatments for some less common diagnoses were discussed previously. If the suspicion for a periodontal etiology is high, anaerobic coverage should be included. Localized cat scratch disease may not routinely require antibiotic therapy and tends to resolve in 2 to 4 months; however, disseminated disease can result which typically requires monotherapy or combination therapy with azithromycin, rifampin, doxycycline, ciprofloxacin, trimethoprim/sulfamethoxazole, or gentamicin, with or without needle aspiration or incision and drainage (10,11). For suspected nontuberculous mycobacteria infection, complete excision of the infected lymph node(s) is optimal, although a 10% risk of facial nerve palsy may be seen (1). Combination therapy with a macrolide plus ethambutol and/or rifampin is an option if the risk for facial nerve injury is too high and excision is not performed.
Questions
1. What are three indications for biopsy of a lymph node?
2. What is the most common cause of acute bilateral cervical lymphadenitis?
3. What is the most common cause of acute unilateral cervical lymphadenitis?
4. What is the most common cause of subacute localized lymphadenitis?
5. What is the most appropriate treatment of suppurative cervical lymphadenitis caused by nontuberculous mycobacteria?
References
1. Healy CM, Baker CJ. Chapter 12. Cervical Lymphadenitis. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ (eds). Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 8th edition. 2018. Elsevier, Philadelphia. pp:124-133.
2. Saxena S, Kumar S, Kharbanda J. Toxoplasmosis submandibular lymphadenitis: Report of an unusual case with a brief review. J Oral Maxillofac Pathol. 2018;22(1):116-120.
3. Haake DA, Levett PN. Chapter 239. Leptospira Species (Leptospirosis) In: Bennett JE, Dolin R, Blaser MJ (eds). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease, 9th edition. 2020. Elsevier, Philadelphia. pp:2898-2905.
4. State of Hawai‘i, Department of Health Disease Outbreak Control Division. Diseases A-Z. https://health.hawaii.gov/docd/diseases-a-z/ Accessed May 2022.
5. Centers for Disease Control and Prevention. More information about the estimated areas with blastomycosis, coccidioidomycosis (Valley fever), and histoplasmosis in the United States. https://www.cdc.gov/fungal/pdf/more-information-about-fungal-maps-508.pdf Accessed May 2022.
6. Centers for Disease Control and Prevention. Monkeypox. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html Accessed June 2022.
7. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox Outbreak — Nine States, May 2022. MMWR Morb Mortal Wkly Rep 2022;71:764–769.
8. Smallpox (Variola). In: American Academy of Pediatrics. Committee on Infectious Diseases, Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH (eds). Red Book: 2021-2024 Report of the Committee on Infectious Diseases, 32nd edition. 2021. Itasca, IL. pp:672-675.
9. Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). https://www.cdc.gov/mis/mis-c/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html Accessed June 2022.
10. Pecora F, Abate L, Scavone S, et al. Management of Infectious Lymphadenitis in Children. Children (Basel). 2021;8(10):860. doi: 10.3390/children8100860
11. Johnson SC, Kosut J, Ching N. Disseminated Cat Scratch Disease in Pediatric Patients in Hawai'i. Hawaii J Health Soc Welf. 2020 May 1;79(5 Suppl 1):64-70.
12. Todd S. Index of Suspicion Case 1 Fever, Neck Swelling, and Weight Loss in a 17-Year-Old Boy. Pediatr Rev 2014;35(10):439–446.
13. Wikner EE, Mulcahy CF, Gitman L, Mudd PA. A 15-year-old with a Slowly Enlarging Submental Mass. Pediatr Rev. 2020 Oct;41(Suppl 1):S50-S53.
14. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Path. 2020;73:697-705.
15. Shetty AK, Beaty MW, McGuirt WF, et al. Kimura’s Disease: A Diagnostic Challenge. Pediatrics. 2002;110(3):e39.
16. Amarilyo G, Rothman D, Manthiram K, et al. CARRA PFAPA Consensus Treatment Plan Workgroup. Consensus treatment plans for periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome (PFAPA): a framework to evaluate treatment responses from the childhood arthritis and rheumatology research alliance (CARRA) PFAPA work group. Pediatr Rheumatol Online J. 2020;15;18(1):31.
17. McClain KL. Peripheral lymphadenopathy in children: Evaluation and diagnostic approach. Drutz, JE, Kaplan SL, Pappo AS (eds). UpToDate. Accessed May 2022.
18. Kuntz M, Kohlfurst DS, Feiterna-Sperling C, et al. NTMkids Consortium. Risk Factors for Complicated Lymphadenitis Caused by Nontuberculous Mycobacteria in Children. Emerg Infect Dis. 2020;26(3):579-586.
19. Desai S, Shah SS, Hall M, et al. Pediatric Research in Inpatient Settings (PRIS) Network. Imaging Strategies and Outcomes in Children Hospitalized with Cervical Lymphadenitis. J Hosp Med. 2020;15(4):197-203.
20. US Food and Drug Administration. FDA recommends thyroid monitoring in babies and young children who receive injections of iodine-containing contrast media for medical imaging https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-thyroid-monitoring-babies-and-young-children-who-receive-injections-iodine-containing#:~:text=Based%20on%20our%20recent%20review,for%20X%2Drays%20and%20other Accessed May 2022.
21. Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55.
22. Weinstock MS, Patel NA, Smith LP. Pediatric Cervical Lymphadenopathy. Pediatr Rev. 2018;39(9):433–443.
Answers to questions
1. Persistent enlargement despite empiric therapy, persistent enlargement or no improvement with negative laboratory work up, rapid growth, matted, fixed, nontender nodes, supraclavicular nodes, fever, weight loss >10%, night sweats.
2. Viral infection
3. Staphylococcus aureus or group A streptococcus
4. Cat scratch disease (Bartonella henselae)
5. Complete surgical excision of the node is both diagnostic and therapeutic.
Return to Table of Contents
University of Hawaii Department of Pediatrics Home Page

