An 8 year-old male with history of a PDA (patent ductus arteriosus) that has not closed presents with an acute onset of decreased movement of his left arm and leg today. The patient states to his mother that it is more difficult to move. There is no pain associated with movement. No recent trauma. He awoke to these symptoms and he did not have them prior to going to sleep. He also states he has had recent mild headaches yesterday. No seizure-like activity has been seen by family and he has no previous history of seizures. Travel history includes the recent direct flight from Hong Kong to Las Vegas during which he slept for most of the flight. Family history is significant for his mother having multiple miscarriages prior to his birth.
Exam T: 37 degrees C, HR 100, RR 22, BP 105/64 mmHg. He is a well-developed, well-nourished male who appears his stated age. He is non-toxic, lying in bed, but appears anxious. Heart exam reveals a grade 2/6 systolic murmur without gallops. Lungs are clear. Facial expression, masetter, tongue, sternocleidomastoid, and trapezius muscle functions are good/symmetric. Extraocular movements are full and conjugate with no nystagmus. Pupils are equal and reactive to light. Funduscopic examination shows sharp optic disc margins and no photophobia. Left upper extremity strength 1/5, left lower extremity strength 2/5, right upper and lower extremity strengths are 4/5. DTRís on the left are +3/4 and left plantar reflex is upgoing with fanning of toes (positive Babinski sign). Right plantar is downgoing.
A CT scan is obtained. It shows an area of mild loss of white-grey differentiation in the right parietal region suggestive of acute ischemic injury. An MRI of the brain shows an area of hypointensity in the right parietal region. An MRA/MRV (magnetic resonance angiogram/venogram) shows a decreased signal distally in the small vessels of the right arterial vasculature suggestive of occlusion versus abnormality of vasculature.
Anticoagulation therapy with low molecular weight heparin (LMWH) is administered subcutaneously every 12 hours with anticipation to complete a six month course of this regimen. In the days prior to discharge, he began to regain gross motor function of his left extremities.
Epidemiology
In North America, 1.2 to 8.0 in 100,000 children per year (1) will have an acute ischemic injury of the brain. Neonates have a greater incidence of stroke at 18 strokes per 100,000 births per year (2). There is a 1.6 to 1 male predominance in neonatal stroke, similar to a 1.5 to 1 male predominance in childhood. This childhood predominance may reflect an increased likelihood for trauma in males (3). Although many of the etiologies of pediatric stroke are unique to pediatrics (arteriopathies, cardiac malformations, and hematologic coagulopathies), the management of these patients is largely taken from the adult population.
Vasculopathies/arteriopathies (see table 1) are present in 50% to 80% of acute ischemic strokes (AIS) (4). Cardiac disease and sickle cell disease are the two most common risk factors. Congenital heart disease was found in 25% of patients with AIS. PFOís (patent foramen ovale) are found in 40% to 50% of patients who experience AIS, as compared to 10% to 27% of the general population. Other cardiac etiologies include cardiac surgery, cardiac catheterization, endocarditis, valvular heart disease, cardiomyopathy, and arrhythmia. Hematologic etiologies include sickle cell disease, iron deficiency anemia, and prothrombotic disorders (coagulopathies). Hypercoagulable states (see table 2) result in abnormal clotting events while hypogocoagulable states place patients at a greater risk for hemorrhagic stroke.
Table 1: Vasculopathies
| Vasculopathy | Description |
|---|
| Moyamoya disease | Progressive occlusive disease of CNS including the circle of Willis. |
| fibromuscular dysplasia | Medium sized arterial angiopathy predominantly affects women of child bearing age. |
| systemic/secondary vasculitis (e.g., Kawasaki disease, Takayasu arteritis) | Small to large vessel vasculitidies with variable CNS involvement. For example, Takayasu has a dirrect effect, while Kawasaki is more of a cardiogenic etiology. |
| arterial infection (bacteremia, bacterial meningitis, etc. | Subarachnoid inflammation seen in bacterial meningitis is related to stroke. 40% with tuberculous meningitis will have AIS. |
| vasospasm | Can be a cause of stroke 4 to 10 days after a subarachnoid hemorrhage. |
| post-varicella angiopathy | Complication of primary varicella-zoster virus infection with approximately 14% of children with a a prothrombotic condition and 86% previously healthy. |
| focal cerebral anteriopathy | Idiopathic arterial stenosis occurring in the brain providing an inciting region for occlusion. |
| congenital arterial abnormalities | Such as focal cerebral arteriopathy but not as iodiopathic since it is congenital. |
| Transient cerebral arteriopathy | Area of arterial injury that may occur due to infection, trauma, or other vascular compromise. |
| childhood primary angiitis of the CNS | Rare reversible inflammatory disease that has not be thoroughly studied in pediatrics. |
A brief review of prothrombotic disorders is displayed in Table 2 below. Patients with sickle cell disease have an increased risk for stroke. It has been reported that silent infarcts can be identified in as many as 28% of asymptomatic children under 6 years old. With the implementation of screening with transcranial Doppler ultrasonography and preventive treatment with chronic red blood cell transfusions, the risk was reduced by over 90% in sickle cell patients (5).
Table 2: Prothrombotic disorders.
| Coagulopathy associated with ischemic stroke | How or Where |
|---|
| deficiency of protein C or activated protein C resistance | Loss of cleaving of Factors Va and VIIIa. Thromboembolism in the venous circulation. |
| deficiency of protein S | Decreased degradation of Factor Va and VIIIa. Thromboembolism in the venous circulation. |
| factor V Leiden mutation or activated protein C (APC) resistance | Point mutation in the factor V gene conferring the resistance of factor V to be inactivated by APC. |
| antithrombin III deficiency | Loss of cleaving thrombin and Factor Xa. Thromboembolism in both arterial and venous systems. |
| prothrombin G20210A mutation | Increased amounts of prothrombin causing increased frequency of clotting. |
| methylenetetrahydrofolate reductase mutations | Increases the amount of homocysteine present in the blood and therefore increases risk of stroke. |
| ATG haplotype of the Z gene | Genetic marker for nonvascular stroke or thromboembolism. This gene expresses a cofactor for the inhibition of Factor Xa. |
| elevated lipoprotein (a) | Correlated to venous thromboembolism. |
| elevated antiphospholipid antibodies | Binds to protein S and inhibits its action. Occurs in both arterial and venous vasculature. |
| elevated factor VIII levels | Stimulate platelet adhesion and thrombus formation in the arterial system. |
| low plasminogen | Decrease in the degradation of fibrin but is unclear if this state needs to coexist with another concurrent condition (e.g., Factor V Leiden). |
| high fibrinogen | Increased levels of fibrinogen correlate with increased venous thrombosis. |
Clinical Presentation
The presentation of a child with an occlusive stroke is dependent on age of the child. In Table 3 below are some age dependent differences in the common presenting signs. Disorders with developmental delays will skew the age of presentation. PedNIHSS (pediatric National Institutes of Health stroke scale) is the pediatric version of the NIH stroke scale (Table 4), which is being evaluated for its ability to accurately measure stroke severity reliably across various developmental ages. If it is able to assess pediatric stroke reliably, it will set the framework for treatment. Lower scores the better.
A good history is a key in the diagnosis. The patientís present illness is important but just as important are birth history and family history. The number of pregnancies and miscarriages of the mother may be the only history of a family clotting disorder. A family tree should be made for other family members with a history of blood clots.
On physical exam, vital signs may show tachycardia and hypertension. These may be related to fear just as much as compensation for reduced cerebral blood flow. The neurological exam should be performed well to assess the approximate location of the stroke. The younger the child, the more difficult this will be.
Table 3: Signs by Age
| Age | Common Presenting Signs |
|---|
| birth to 6 months old | Seizures are presenting sign in 80%. Other signs include apnea, irritability, jitteriness, and lethargy. |
| 6 months old to 1 year | Either seizures or focal signs and hemiparesis. |
| 1 year old and above | Focal motor signs. |
Table 4: PedNIHSS (abbreviated form)
| Item | Sub-Class | Scoring |
|---|
| LOC | A General | 0-3: alert to unresponsive or reflex motor |
| | B Question | 0-2: 2 questions correct to neither correct |
|---|
| | C Command | 0-2: 2 correct performances to none |
|---|
| Best Gaze | | 0-2: normal to forced deviation or paresis |
| Visual | | 0-3: no loss to bilateral hemianopia |
| Facial Palsy | | 0-3: normal to complete paralysis of one or both sides of face |
| Extremities | Upper | 0-4: normal to no movement, a 9 is given for amputation or explanation needed |
| | Lower | 0-4: normal to no movement, a 9 is given for amputation or explanation needed |
|---|
| Limb Ataxia | | 0-2: absent to present in 2 limbs |
| Sensory | | 0-2: normal to severe/total sensory loss |
| Best Language | | 0-3: no aphasia to mute/global aphasia |
Labs and Imaging
Imaging is of critical importance in the diagnostic workup for stroke. Magnetic resonance imaging (MRI), specifically T2, DWI (diffusion weighted imaging), and FLAIR (fluid attenuated inversion recovery) imaging, has traditionally been used in the evaluation of strokes. In adults, DWI has been reported to detect >90% of acute ischemic lesions in the brain (6). Diffusion weighted images have the ability to detect ischemia within hours whereas a CT may not be able to detect ischemia for 24 hours. Magnetic resonance angiography (MRA) has better sensitivity and specificity with extracranial vascular abnormalities when compared with intracranial abnormalities. Magnetic resonance venography (MRV) can be used to diagnose cerebral venous thrombosis. Newer CT angiography and venography (CTA and CTV) can reduce the amount of ionizing radiation delivered during imaging. Current CT technology offers superb visualization of vascular structures, but requires the subject to remain still and proper timing of the contrast injection. If there is history that suggests hemorrhage, a CT scan is a quicker technique in identifying size and location. Another imaging modality that has been studied is ultrasound which is commonly used in neonates to evaluate for IVH (intraventricular hemorrhage) and PVL (periventricular leukomalacia). However, it has not been show, to reliably assess stroke (7).
Laboratory testing is based on the history and physical examination. When a hemorrhagic stroke is identified, standard laboratory tests to evaluate for bleeding disorders are obtained in addition to any other tests that pertain to the patientís clinical presentation. The tables below provide the recommended tests and divide some of these tests by acquired versus congenital risk factors.
Table 5: Laboratory Tests
| Initial Tests | CBC including platelets
comprehensive chemistry panel including liver enzymes
ESR and CRP
DIC panel (PT/INR/aPTT, D-dimer, fibrinogen
|
|---|
| Congenital Risk Factor Tests | thrombophilia panel (see Table 6 below)
metabolic disease screening
mitochondrial DNA mutation analyses
|
|---|
| Acquired Risk Factor Tests | viral infection evaluation
urine toxicology screen
ANA
|
|---|
Table 6: Thrombophilia Panel
| protein C and S antigen and activity levels |
| factor V Leiden mutation |
| antithrombin activity |
| factor VIII activity |
| prothrombin G20210A mutation |
| homocysteine concentration (+/- MTHFR) |
| antiphospholipid antibody |
| lipoprotein (a) concentration |
| lupus anticoagulant |
| anticardiolipin IgG and IgM |
| anti-beta-2-glycoprotein I IgG and IgM |
Cardiac evaluation begins with an EKG to assess for arrhythmia. Echocardiography is often performed to evaluate for undetected congenital heart disease due to their increased risks of AIS. Transthoracic echocardiogram with agitated saline (producing bubble artifact) is a specific test that has been used to assess for a patent foramen ovale. The release of venous thrombi can exploit this bypass to deposit in the cerebral vasculature.
Treatment and Prognosis
The American College of Chest Physicians provides guidelines in treating pediatric ischemic stroke. However, the literature is lacking studies specific to the pediatric population and much of the guidelines are extrapolated from the results obtained in adults, including proper supportive care.
Antithrombotic therapy is still in its infancy of research for the pediatric population. Current treatment is taken from adult stroke guidelines or based on consensus panels (8). Antithrombotic therapy has the risk of associated intracranial hemorrhage. In the International Pediatric Stroke Study, the overall rate of intracranial hemorrhage was 5%. This study also reported an overall acute mortality rate of 3% for childhood acute ischemic stroke. Early studies on thrombolytics show effective reduction of clot but a majority of patients have still retained neurologic deficits at time of discharge. The data was not distinguished by the specific etiology of the stroke and therefore no comparisons were made between the different origins of stroke.
Surgically, advances in mechanical recanalization are currently undergoing clinical trials for the adult population. At least one of these trials has incorporated pediatric patients using the same guidelines as their adult counterparts of the trial.
Early evacuation of hemorrhagic products, as compared to medical management, has been shown to not have an effect on the outcome of hemorrhagic stroke in the older population (9). However in adults less than 45 years of age, it has been reported that early surgery can be beneficial. Further studies, especially in the pediatric population are needed.
The data for the support of rehabilitative services for pediatric stroke is lacking; however, the general consensus and standard of care is to proceed with intensive therapy specific to the childís needs. As discussed earlier, PedNIHSS, if confirmed as reliable, will be a key factor in being able to evaluate various modalities implemented in the treatment of pediatric stroke.
Neurologic sequelae of childhood stroke falls into three major categories: neuromotor, language, and cognitive. The age when the stroke was diagnosed, the size and locations of the stroke, and comorbid conditions all lend to the high variability of outcomes. Among the reported results of childhood ischemic strokes, 30% of survivors have normal motor function when evaluated at 6 months to 2.5 years after the initial insult (2). It was also noted that incidence of seizures following childhood strokes are 25% to 33% at 5 to 7 years after the stroke. The outcomes of childhood stroke are more variable than neonatal stroke. Cognitive and behavioral deficits were found in 3% to 14% of patients at 2 to 6 years after the neonatal ischemic stroke.
Recurrence risks vary between 20% to 40% for patients with pediatric non-neonatal acute ischemic stroke at 5 years and up to 3% in neonatal acute ischemic stroke at 3.5 to 6 years. This risk is largely dependent on the underlying risk factors for each specific patient. The more risk factors a patient has, the greater the risk of recurrence. Of the risk factors taken as a single entity, vasculopathy is shown to have a significant increased risk of recurrence in a large multi-center prospective study (2).
Conclusion
Pediatric stroke is a rare entity in pediatrics. The signs and symptoms of stroke can be largely varied and stroke should be part of the differential of many abnormal neurologic presentations. For children, there is much to be learned about appropriate management, as much of our understanding comes from adult studies. The sequelae of stroke can be devastating to patient and parent. Education and support are very important components of medicine that we can offer during the initial event.
Questions
1. What are the 2 most common risk factors for pediatric ischemic stroke?
2. Describe the most common presenting sign in infants and how it changes with age?
3. What are the 3 categories of neurologic sequelae that befall a child with a stroke?
4. What would be the best diagnostic test for a child suspected of having a stroke?
5. True or False. Children have a greater incidence of stroke than neonates
Related X-rays
The angiographic images below are from: Brown KR, Yamamoto LG. Moyamoya Disease. In: Yamamoto LG, Inaba AS, DiMauro R (eds). Radiology Cases In Pediatric Emergency Medicine, 1996, volume 3, case 9. Read the online case and description at: www.hawaii.edu/medicine/pediatrics/pemxray/v3c09.html
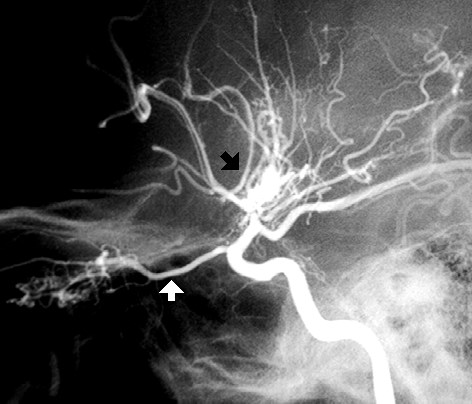
This an internal carotid angiogram of a patient with Moyamoya disease. The black arrow points at the "puff of smoke" which represents neovascularization providing collateral blood flow. There is stenosis of the internal carotid artery proximal to this puff of smoke. The white arrow points to a dilated ophthalmic artery which is providing collateral
circulation as well.
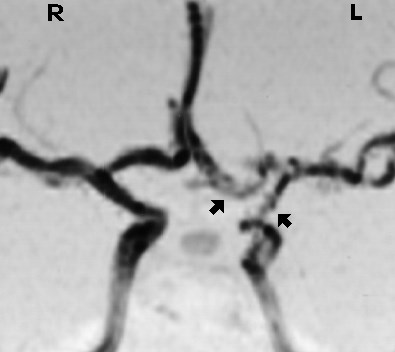
This is a magnetic resonance angiogram of a patient with fibromuscular dysplasia. The arrows point at narrowing and irregularity of the left internal carotid artery and its branches.
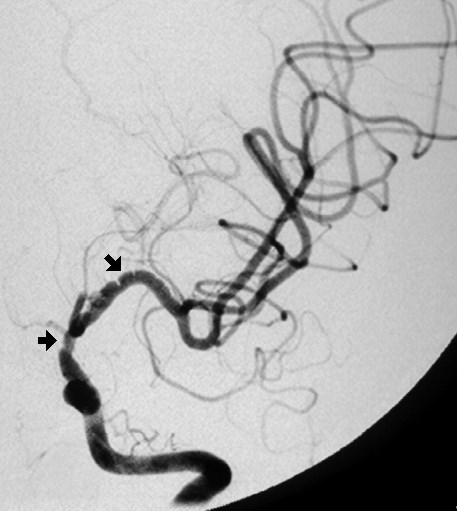
This an AP view of a left internal carotid angiogram. The arrows point to narrowed regions of the internal carotid artery and its branches in a patient with fibromuscular dysplasia.
Family Education
1. http://www.pediatricstroke.org/
2. http://www.stroke.org/site/PageServer?pagename=PEDSTROKE
3. http://www.strokeassociation.org/STROKEORG/AboutStroke/StrokeInChildren/Stroke-In-Children_UCM_308543_SubHomePage.jsp
References
1. Agrawal N, et al. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke 2009;40(11):3415-3421.
2. Bernard TJ, Goldenberg NA. Pediatric arterial ischemic stroke. Pediatric Clinics of North America 2008;55(2):323-338.
3. Golomb MR, Fullerton HJ, Nowak-Gottl U. Male predominance in childhood ischemic stroke findings from the International Pediatric Stroke Study. Stroke 2009;40(1):52-57.
4. Amlie-Lefond C, et al. Predictors of Cerebral Arteriopathy in Children With Arterial Ischemic Stroke Results of the International Pediatric Stroke Study. Circulation 2009;119(10):1417-1423.
5. Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15(5):344-349.
6. Alberts MJ, et al. Recommendations for Comprehensive Stroke Centers A Consensus Statement From the Brain Attack Coalition. Stroke 2005;36(7):1597-1616.
7. Mackay MT, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Annals of Neurology 2011;69(1):130-140.
8. Goldenberg NA, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurology 2009;8(12):1120-1127.
9. Mendelow AD, et al. Early Surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haemorrhage (STICH): a randomized trial. Lancet 2005;365:387-397.
Answers to questions
1. Cardiac disease and sickle cell disease.
2. Infants present most often with seizures and as children become older they present with more and more focal symptoms.
3. Cognitive, language, or neuromotor. Each presentation creates a different pattern of injury and degree of recovery. Families may be frustrated by the lack of a simple answer. It is important to care for the patient but also make sure the family has support as well.
4. MRI of the brain with and without contrast is the best diagnostic study. However, pediatric stroke, like most illnesses, is often best understood by obtaining a proper history.
5. False. Neonates have an incidence of 18 strokes per 100,000 births per year.
Return to Table of Contents
University of Hawaii Department of Pediatrics Home Page



