Pulmonary Sequestration
Radiology Cases in Pediatric Emergency Medicine
Volume 5, Case 14
Craig T. Nakamura, MD
Kapiolani Medical Center For Women And Children
University of Hawaii John A. Burns School of Medicine
This is a 13 month old male brought to the
emergency department with wheezing, coughing, and
rhinorrhea. He has had these symptoms for the past
month. Tonight, he developed fever which prompted
his parents to bring him to the E.D. He was seen by his
primary care physician three weeks ago. A chest
radiograph was obtained on that day, revealing a left
lower lobe consolidation. He was treated with albuterol
syrup and a ten day course of clarithromycin with some
improvement. He was noted to have a poor appetite
and lost approximately one kilogram over the course of
the month.
Past medical history. He was born at 39 weeks
gestation without complications. In the nursery, he was
noted to be tachypneic with subcostal retractions. A
chest radiograph in the nursery revealed a left lower
lobe infiltrate. He was treated with oxygen and
intravenous antibiotics. He was then discharged home
after one week. Over the first year of his life, he was
seen by his pediatrician on seven occasions for upper
respiratory tract infections.
Exam in the E.D.: T 37.5 degrees rectally, P 138,
RR 52, BP 95/40, oxygen saturation in room air 95%.
General appearance: Responsive with diminished
activity in mild respiratory distress. HEENT: Normal
except for white rhinorrhea. Neck without adenopathy.
Lungs clear to auscultation bilaterally. Breath sounds
were diminished at the left base. There were no
wheezes, rhonchi, or rales. He has mild subcostal
retractions and a paroxsymal cough. Heart regular
without murmurs. Abdomen benign. Color and
perfusion are good
Labs in the E.D.: Hgb 9.6, hct 30.0, WBC 30,600
with a differential of 48% segs, 13% bands, 33%
lymphs and 6% monos. Platelet count 518,000. A
blood culture is drawn. A chest radiograph is obtained.
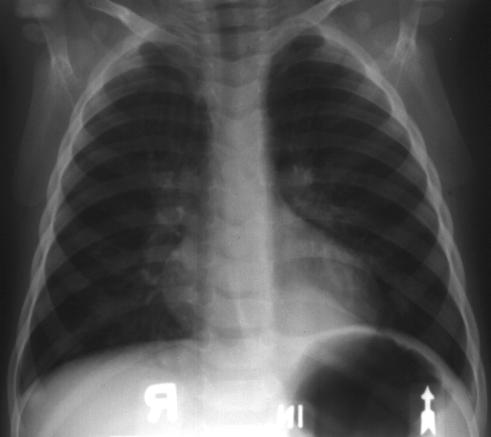
View CXR [PA view]
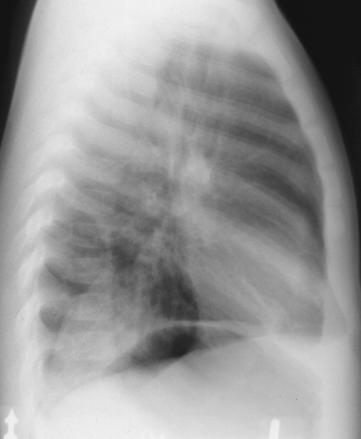
 [Lateral view]
[Lateral view]
 The PA view demonstrates a left sided triangular
density of the medial left lung base.
On the lateral view, the triangular density is seen
posteriorly over the left lung base. Usually, the right
diaphragm is higher than the left diaphragm. In this
case, the left diaphragm, which is higher than the right
diaphragm, can be identified as the diaphragm with the
underlying gastric bubble. In this lateral view, the
density can be determined to be on the left side.
View density.
The PA view demonstrates a left sided triangular
density of the medial left lung base.
On the lateral view, the triangular density is seen
posteriorly over the left lung base. Usually, the right
diaphragm is higher than the left diaphragm. In this
case, the left diaphragm, which is higher than the right
diaphragm, can be identified as the diaphragm with the
underlying gastric bubble. In this lateral view, the
density can be determined to be on the left side.
View density.
 The patient is hospitalized and treated with
intravenous antibiotics. The history of recurrent
pulmonary infections suggests the possibility of a
pulmonary anomaly. An aortogram is performed.
View aortogram.
The patient is hospitalized and treated with
intravenous antibiotics. The history of recurrent
pulmonary infections suggests the possibility of a
pulmonary anomaly. An aortogram is performed.
View aortogram.
 This aortogram shows contrast injected into the
aortic arch. There is a large anomalous vessel from the
infradiaphragmatic portion of the aorta that supplies the
abnormal density at the left lung base. The venous
phase (not shown) revealed drainage into the
hemiazygous vein (a systemic vein). This abnormal
vascular supply is indicative of a pulmonary
sequestration.
Discussion
Pulmonary sequestration (PS) as first described by
Rektorzik in 1861 is a mass of accessory lung tissue
with an anomalous arterial supply. The pulmonary
tissue is dysplastic and nonfunctioning without any
connection to the tracheobronchial tree (1). The
etiology of this defect is thought to be congenital (2).
There are two types of pulmonary sequestration:
intralobar and extralobar.
Intralobar PS is three to six times more common
than the extralobar type (3). In intralobar PS, the
pulmonary tissue is isolated from the normal lung
tissue; however, the pleural covering remains
contiguous with that of the lung. The left lung is
involved in 65% of the cases (4). Typically, the mass is
confined to the posterior basilar segments of the lower
lobe of the lung. There are rarely associated anomalies
or foregut communications. The symptoms typically
occur during early childhood with the patient presenting
with recurrent pneumonia. The diagnosis is made after
the age of 20 years in fifty percent of this type of PS
(5). The incidence of intralobar PS is equal in males
and females (6). The arterial supply is via a systemic
artery and the venous drainage is through the
pulmonary veins.
The accessory lung tissue of extralobar PS is
contained within its own pleural sac and is separated
from the rest of the lung. It may be located between
the inferior surface of the lower lobe and diaphragm,
below the diaphragm, within the diaphragm, or in the
mediastinum. It occurs on the left in greater than 90%
of the cases (5). There may be an occasional foregut
communication and associated anomalies are quite
common. These may consist of a diaphragmatic
hernia, cardiovascular malformation, bronchogenic cyst,
pectus excavatum, or other lung anomalies (4). In
contrast to intralobar PS, extralobar PS is usually
diagnosed in infancy secondary to respiratory distress
or feeding difficulties. Since the accessory tissue is
sequestered within its own pleura, the chances of
presentation with an infection are less than that of
intralobar PS, unless there is a foregut communication.
The arterial supply is from a systemic artery and the
venous drainage is typically via the systemic veins,
rather than the pulmonary veins as seen in intralobar
PS.
Most radiographically visible sequestrations occur in
children over one year of age. The appearance of the
chest radiograph depends on several factors: 1)
whether the lesion is a site of infection, 2) if there is a
communication with the airway or contiguous lung
tissue, and 3) if there are other associated lung
anomalies (7). Intralobar sequestration typically
appears as a mass, cystic lesion, or infiltrative shadow
with ill-defined borders. The majority of extralobar
sequestrations are small lesions and are not visible on
chest radiographs. However, they may present as an
infiltrate or mass in the region between the lower lobes
and the diaphragm (but can also be found in the
superior or anterior mediastinum, pericardium, or
infradiaphragmatic region).
In the diagnosis of pulmonary sequestration, a CT,
MRI, or ultrasound may be diagnostic. However, a
normal study does not exclude the diagnosis. The gold
standard for identifying a sequestration is angiography
(7). Angiography confirms the anatomy, identifies the
systemic supply, and shows the venous drainage.
It is now thought that there are many "variants" to
the pulmonary sequestration spectrum (8,9) which
include: scimitar syndrome, horseshoe lung, cystic
adenomatoid malformation, and pulmonary
arteriovenous fistula/malformation.
In the scimitar syndrome, the anomalous vein drains
into the inferior vena cava or at its junction at the right
atrium. This vein has the appearance of a scimitar.
This may or may not be accompanied by hypoplasia of
the right lung and dextrocardia, anomalies of the lobes
of the right lung, hypoplasia of the right pulmonary
artery, and an anomalous systemic vascular supply to
the lung (10).
The horseshoe lung is a rare congenital anomaly. It
is associated with some of the findings of the scimitar
syndrome. There is an isthmus of pulmonary tissue
which extends from the right lung base across the
midline behind the pericardium and then fuses with the
left lung base. Likewise, there may be an anomalous
systemic supply (1).
The cystic adenomatoid malformation is an
abnormality of the pulmonary parenchyma due to an
overgrowth of bronchioles (1). There is usually a normal
vascular supply, however there may be an aberrant
systemic artery.
Lastly, the pulmonary arteriovenous
fistula/malformation consists of an abnormal pulmonary
artery and venous connection (1). In this condition,
there is normal pulmonary parenchyma (1) .
Regardless of which variant is present, a diagnosis is
suggested clinically and confirmed with angiography.
Bibliography:
1. Felker RE, Tonkin ILD. Imaging of Pulmonary
Sequestration. AJR. 1990;154:241-249.
2. Nicolette LA, Kosloske AM, Bartow SA, Murphy
S. Intralobar Pulmonary sequestration: a clinical and
pathological spectrum. Journal of Pediatric Surgery
1993;28(6):802-805.
3. Sugio K, Kaneko S, Yokoyama H, Ishida T,
Sugimachi K, Hasuo K. Pulmonary sequestration in
older child and in adults. Int Surg 1992;77:102-107.
4. Javaid A, Aamir AUH. Pulmonary sequestration:
a case report and review. Respiratory Medicine
1994;88:65-66.
5. Lin CH, Lin CT, Chen CY, Peng HC, Chen HC,
Wang PY. Pulmonary sequestration. Chin Med J
(Taipei) 1994;53:168-172.
6. Savic B, Birtel FJ, Knoche R, et al. Pulmonary
Sequestration. In: Frick HP, Harnack GA, Martini GA
et al (eds). Advances in Internal Medicine and
Pediatrics. New York, Springer-Verlag, 1979, pp.
58-92.
7. John PR, Beasley SW, Mayne V. Pulmonary
sequestration and related congenital disorders: A
clinico-radiological review of 41 cases. Pediatr Radiol
1989;20:4-9.
8. Louie HWt Martin SM, Mulder DG. Pulmonary
sequestration: 17-year Experience at UCLA. The
American Surgeon 1993;59:801-805.
9. Clements BS, Warner J. Pulmonary
sequestration and related congenital
bronchopulmonary-vascular malformations:
Nomenclature and classification based on anatomical
and embryological considerations. Thorax
1987;42:401-408.
This aortogram shows contrast injected into the
aortic arch. There is a large anomalous vessel from the
infradiaphragmatic portion of the aorta that supplies the
abnormal density at the left lung base. The venous
phase (not shown) revealed drainage into the
hemiazygous vein (a systemic vein). This abnormal
vascular supply is indicative of a pulmonary
sequestration.
Discussion
Pulmonary sequestration (PS) as first described by
Rektorzik in 1861 is a mass of accessory lung tissue
with an anomalous arterial supply. The pulmonary
tissue is dysplastic and nonfunctioning without any
connection to the tracheobronchial tree (1). The
etiology of this defect is thought to be congenital (2).
There are two types of pulmonary sequestration:
intralobar and extralobar.
Intralobar PS is three to six times more common
than the extralobar type (3). In intralobar PS, the
pulmonary tissue is isolated from the normal lung
tissue; however, the pleural covering remains
contiguous with that of the lung. The left lung is
involved in 65% of the cases (4). Typically, the mass is
confined to the posterior basilar segments of the lower
lobe of the lung. There are rarely associated anomalies
or foregut communications. The symptoms typically
occur during early childhood with the patient presenting
with recurrent pneumonia. The diagnosis is made after
the age of 20 years in fifty percent of this type of PS
(5). The incidence of intralobar PS is equal in males
and females (6). The arterial supply is via a systemic
artery and the venous drainage is through the
pulmonary veins.
The accessory lung tissue of extralobar PS is
contained within its own pleural sac and is separated
from the rest of the lung. It may be located between
the inferior surface of the lower lobe and diaphragm,
below the diaphragm, within the diaphragm, or in the
mediastinum. It occurs on the left in greater than 90%
of the cases (5). There may be an occasional foregut
communication and associated anomalies are quite
common. These may consist of a diaphragmatic
hernia, cardiovascular malformation, bronchogenic cyst,
pectus excavatum, or other lung anomalies (4). In
contrast to intralobar PS, extralobar PS is usually
diagnosed in infancy secondary to respiratory distress
or feeding difficulties. Since the accessory tissue is
sequestered within its own pleura, the chances of
presentation with an infection are less than that of
intralobar PS, unless there is a foregut communication.
The arterial supply is from a systemic artery and the
venous drainage is typically via the systemic veins,
rather than the pulmonary veins as seen in intralobar
PS.
Most radiographically visible sequestrations occur in
children over one year of age. The appearance of the
chest radiograph depends on several factors: 1)
whether the lesion is a site of infection, 2) if there is a
communication with the airway or contiguous lung
tissue, and 3) if there are other associated lung
anomalies (7). Intralobar sequestration typically
appears as a mass, cystic lesion, or infiltrative shadow
with ill-defined borders. The majority of extralobar
sequestrations are small lesions and are not visible on
chest radiographs. However, they may present as an
infiltrate or mass in the region between the lower lobes
and the diaphragm (but can also be found in the
superior or anterior mediastinum, pericardium, or
infradiaphragmatic region).
In the diagnosis of pulmonary sequestration, a CT,
MRI, or ultrasound may be diagnostic. However, a
normal study does not exclude the diagnosis. The gold
standard for identifying a sequestration is angiography
(7). Angiography confirms the anatomy, identifies the
systemic supply, and shows the venous drainage.
It is now thought that there are many "variants" to
the pulmonary sequestration spectrum (8,9) which
include: scimitar syndrome, horseshoe lung, cystic
adenomatoid malformation, and pulmonary
arteriovenous fistula/malformation.
In the scimitar syndrome, the anomalous vein drains
into the inferior vena cava or at its junction at the right
atrium. This vein has the appearance of a scimitar.
This may or may not be accompanied by hypoplasia of
the right lung and dextrocardia, anomalies of the lobes
of the right lung, hypoplasia of the right pulmonary
artery, and an anomalous systemic vascular supply to
the lung (10).
The horseshoe lung is a rare congenital anomaly. It
is associated with some of the findings of the scimitar
syndrome. There is an isthmus of pulmonary tissue
which extends from the right lung base across the
midline behind the pericardium and then fuses with the
left lung base. Likewise, there may be an anomalous
systemic supply (1).
The cystic adenomatoid malformation is an
abnormality of the pulmonary parenchyma due to an
overgrowth of bronchioles (1). There is usually a normal
vascular supply, however there may be an aberrant
systemic artery.
Lastly, the pulmonary arteriovenous
fistula/malformation consists of an abnormal pulmonary
artery and venous connection (1). In this condition,
there is normal pulmonary parenchyma (1) .
Regardless of which variant is present, a diagnosis is
suggested clinically and confirmed with angiography.
Bibliography:
1. Felker RE, Tonkin ILD. Imaging of Pulmonary
Sequestration. AJR. 1990;154:241-249.
2. Nicolette LA, Kosloske AM, Bartow SA, Murphy
S. Intralobar Pulmonary sequestration: a clinical and
pathological spectrum. Journal of Pediatric Surgery
1993;28(6):802-805.
3. Sugio K, Kaneko S, Yokoyama H, Ishida T,
Sugimachi K, Hasuo K. Pulmonary sequestration in
older child and in adults. Int Surg 1992;77:102-107.
4. Javaid A, Aamir AUH. Pulmonary sequestration:
a case report and review. Respiratory Medicine
1994;88:65-66.
5. Lin CH, Lin CT, Chen CY, Peng HC, Chen HC,
Wang PY. Pulmonary sequestration. Chin Med J
(Taipei) 1994;53:168-172.
6. Savic B, Birtel FJ, Knoche R, et al. Pulmonary
Sequestration. In: Frick HP, Harnack GA, Martini GA
et al (eds). Advances in Internal Medicine and
Pediatrics. New York, Springer-Verlag, 1979, pp.
58-92.
7. John PR, Beasley SW, Mayne V. Pulmonary
sequestration and related congenital disorders: A
clinico-radiological review of 41 cases. Pediatr Radiol
1989;20:4-9.
8. Louie HWt Martin SM, Mulder DG. Pulmonary
sequestration: 17-year Experience at UCLA. The
American Surgeon 1993;59:801-805.
9. Clements BS, Warner J. Pulmonary
sequestration and related congenital
bronchopulmonary-vascular malformations:
Nomenclature and classification based on anatomical
and embryological considerations. Thorax
1987;42:401-408.
Return to Radiology Cases In Ped Emerg Med Case Selection Page
Return to Univ. Hawaii Dept. Pediatrics Home Page
 [Lateral view]
[Lateral view]
 The PA view demonstrates a left sided triangular
density of the medial left lung base.
On the lateral view, the triangular density is seen
posteriorly over the left lung base. Usually, the right
diaphragm is higher than the left diaphragm. In this
case, the left diaphragm, which is higher than the right
diaphragm, can be identified as the diaphragm with the
underlying gastric bubble. In this lateral view, the
density can be determined to be on the left side.
View density.
The PA view demonstrates a left sided triangular
density of the medial left lung base.
On the lateral view, the triangular density is seen
posteriorly over the left lung base. Usually, the right
diaphragm is higher than the left diaphragm. In this
case, the left diaphragm, which is higher than the right
diaphragm, can be identified as the diaphragm with the
underlying gastric bubble. In this lateral view, the
density can be determined to be on the left side.
View density.
 The patient is hospitalized and treated with
intravenous antibiotics. The history of recurrent
pulmonary infections suggests the possibility of a
pulmonary anomaly. An aortogram is performed.
View aortogram.
The patient is hospitalized and treated with
intravenous antibiotics. The history of recurrent
pulmonary infections suggests the possibility of a
pulmonary anomaly. An aortogram is performed.
View aortogram.
 This aortogram shows contrast injected into the
aortic arch. There is a large anomalous vessel from the
infradiaphragmatic portion of the aorta that supplies the
abnormal density at the left lung base. The venous
phase (not shown) revealed drainage into the
hemiazygous vein (a systemic vein). This abnormal
vascular supply is indicative of a pulmonary
sequestration.
Discussion
Pulmonary sequestration (PS) as first described by
Rektorzik in 1861 is a mass of accessory lung tissue
with an anomalous arterial supply. The pulmonary
tissue is dysplastic and nonfunctioning without any
connection to the tracheobronchial tree (1). The
etiology of this defect is thought to be congenital (2).
There are two types of pulmonary sequestration:
intralobar and extralobar.
Intralobar PS is three to six times more common
than the extralobar type (3). In intralobar PS, the
pulmonary tissue is isolated from the normal lung
tissue; however, the pleural covering remains
contiguous with that of the lung. The left lung is
involved in 65% of the cases (4). Typically, the mass is
confined to the posterior basilar segments of the lower
lobe of the lung. There are rarely associated anomalies
or foregut communications. The symptoms typically
occur during early childhood with the patient presenting
with recurrent pneumonia. The diagnosis is made after
the age of 20 years in fifty percent of this type of PS
(5). The incidence of intralobar PS is equal in males
and females (6). The arterial supply is via a systemic
artery and the venous drainage is through the
pulmonary veins.
The accessory lung tissue of extralobar PS is
contained within its own pleural sac and is separated
from the rest of the lung. It may be located between
the inferior surface of the lower lobe and diaphragm,
below the diaphragm, within the diaphragm, or in the
mediastinum. It occurs on the left in greater than 90%
of the cases (5). There may be an occasional foregut
communication and associated anomalies are quite
common. These may consist of a diaphragmatic
hernia, cardiovascular malformation, bronchogenic cyst,
pectus excavatum, or other lung anomalies (4). In
contrast to intralobar PS, extralobar PS is usually
diagnosed in infancy secondary to respiratory distress
or feeding difficulties. Since the accessory tissue is
sequestered within its own pleura, the chances of
presentation with an infection are less than that of
intralobar PS, unless there is a foregut communication.
The arterial supply is from a systemic artery and the
venous drainage is typically via the systemic veins,
rather than the pulmonary veins as seen in intralobar
PS.
Most radiographically visible sequestrations occur in
children over one year of age. The appearance of the
chest radiograph depends on several factors: 1)
whether the lesion is a site of infection, 2) if there is a
communication with the airway or contiguous lung
tissue, and 3) if there are other associated lung
anomalies (7). Intralobar sequestration typically
appears as a mass, cystic lesion, or infiltrative shadow
with ill-defined borders. The majority of extralobar
sequestrations are small lesions and are not visible on
chest radiographs. However, they may present as an
infiltrate or mass in the region between the lower lobes
and the diaphragm (but can also be found in the
superior or anterior mediastinum, pericardium, or
infradiaphragmatic region).
In the diagnosis of pulmonary sequestration, a CT,
MRI, or ultrasound may be diagnostic. However, a
normal study does not exclude the diagnosis. The gold
standard for identifying a sequestration is angiography
(7). Angiography confirms the anatomy, identifies the
systemic supply, and shows the venous drainage.
It is now thought that there are many "variants" to
the pulmonary sequestration spectrum (8,9) which
include: scimitar syndrome, horseshoe lung, cystic
adenomatoid malformation, and pulmonary
arteriovenous fistula/malformation.
In the scimitar syndrome, the anomalous vein drains
into the inferior vena cava or at its junction at the right
atrium. This vein has the appearance of a scimitar.
This may or may not be accompanied by hypoplasia of
the right lung and dextrocardia, anomalies of the lobes
of the right lung, hypoplasia of the right pulmonary
artery, and an anomalous systemic vascular supply to
the lung (10).
The horseshoe lung is a rare congenital anomaly. It
is associated with some of the findings of the scimitar
syndrome. There is an isthmus of pulmonary tissue
which extends from the right lung base across the
midline behind the pericardium and then fuses with the
left lung base. Likewise, there may be an anomalous
systemic supply (1).
The cystic adenomatoid malformation is an
abnormality of the pulmonary parenchyma due to an
overgrowth of bronchioles (1). There is usually a normal
vascular supply, however there may be an aberrant
systemic artery.
Lastly, the pulmonary arteriovenous
fistula/malformation consists of an abnormal pulmonary
artery and venous connection (1). In this condition,
there is normal pulmonary parenchyma (1) .
Regardless of which variant is present, a diagnosis is
suggested clinically and confirmed with angiography.
Bibliography:
1. Felker RE, Tonkin ILD. Imaging of Pulmonary
Sequestration. AJR. 1990;154:241-249.
2. Nicolette LA, Kosloske AM, Bartow SA, Murphy
S. Intralobar Pulmonary sequestration: a clinical and
pathological spectrum. Journal of Pediatric Surgery
1993;28(6):802-805.
3. Sugio K, Kaneko S, Yokoyama H, Ishida T,
Sugimachi K, Hasuo K. Pulmonary sequestration in
older child and in adults. Int Surg 1992;77:102-107.
4. Javaid A, Aamir AUH. Pulmonary sequestration:
a case report and review. Respiratory Medicine
1994;88:65-66.
5. Lin CH, Lin CT, Chen CY, Peng HC, Chen HC,
Wang PY. Pulmonary sequestration. Chin Med J
(Taipei) 1994;53:168-172.
6. Savic B, Birtel FJ, Knoche R, et al. Pulmonary
Sequestration. In: Frick HP, Harnack GA, Martini GA
et al (eds). Advances in Internal Medicine and
Pediatrics. New York, Springer-Verlag, 1979, pp.
58-92.
7. John PR, Beasley SW, Mayne V. Pulmonary
sequestration and related congenital disorders: A
clinico-radiological review of 41 cases. Pediatr Radiol
1989;20:4-9.
8. Louie HWt Martin SM, Mulder DG. Pulmonary
sequestration: 17-year Experience at UCLA. The
American Surgeon 1993;59:801-805.
9. Clements BS, Warner J. Pulmonary
sequestration and related congenital
bronchopulmonary-vascular malformations:
Nomenclature and classification based on anatomical
and embryological considerations. Thorax
1987;42:401-408.
This aortogram shows contrast injected into the
aortic arch. There is a large anomalous vessel from the
infradiaphragmatic portion of the aorta that supplies the
abnormal density at the left lung base. The venous
phase (not shown) revealed drainage into the
hemiazygous vein (a systemic vein). This abnormal
vascular supply is indicative of a pulmonary
sequestration.
Discussion
Pulmonary sequestration (PS) as first described by
Rektorzik in 1861 is a mass of accessory lung tissue
with an anomalous arterial supply. The pulmonary
tissue is dysplastic and nonfunctioning without any
connection to the tracheobronchial tree (1). The
etiology of this defect is thought to be congenital (2).
There are two types of pulmonary sequestration:
intralobar and extralobar.
Intralobar PS is three to six times more common
than the extralobar type (3). In intralobar PS, the
pulmonary tissue is isolated from the normal lung
tissue; however, the pleural covering remains
contiguous with that of the lung. The left lung is
involved in 65% of the cases (4). Typically, the mass is
confined to the posterior basilar segments of the lower
lobe of the lung. There are rarely associated anomalies
or foregut communications. The symptoms typically
occur during early childhood with the patient presenting
with recurrent pneumonia. The diagnosis is made after
the age of 20 years in fifty percent of this type of PS
(5). The incidence of intralobar PS is equal in males
and females (6). The arterial supply is via a systemic
artery and the venous drainage is through the
pulmonary veins.
The accessory lung tissue of extralobar PS is
contained within its own pleural sac and is separated
from the rest of the lung. It may be located between
the inferior surface of the lower lobe and diaphragm,
below the diaphragm, within the diaphragm, or in the
mediastinum. It occurs on the left in greater than 90%
of the cases (5). There may be an occasional foregut
communication and associated anomalies are quite
common. These may consist of a diaphragmatic
hernia, cardiovascular malformation, bronchogenic cyst,
pectus excavatum, or other lung anomalies (4). In
contrast to intralobar PS, extralobar PS is usually
diagnosed in infancy secondary to respiratory distress
or feeding difficulties. Since the accessory tissue is
sequestered within its own pleura, the chances of
presentation with an infection are less than that of
intralobar PS, unless there is a foregut communication.
The arterial supply is from a systemic artery and the
venous drainage is typically via the systemic veins,
rather than the pulmonary veins as seen in intralobar
PS.
Most radiographically visible sequestrations occur in
children over one year of age. The appearance of the
chest radiograph depends on several factors: 1)
whether the lesion is a site of infection, 2) if there is a
communication with the airway or contiguous lung
tissue, and 3) if there are other associated lung
anomalies (7). Intralobar sequestration typically
appears as a mass, cystic lesion, or infiltrative shadow
with ill-defined borders. The majority of extralobar
sequestrations are small lesions and are not visible on
chest radiographs. However, they may present as an
infiltrate or mass in the region between the lower lobes
and the diaphragm (but can also be found in the
superior or anterior mediastinum, pericardium, or
infradiaphragmatic region).
In the diagnosis of pulmonary sequestration, a CT,
MRI, or ultrasound may be diagnostic. However, a
normal study does not exclude the diagnosis. The gold
standard for identifying a sequestration is angiography
(7). Angiography confirms the anatomy, identifies the
systemic supply, and shows the venous drainage.
It is now thought that there are many "variants" to
the pulmonary sequestration spectrum (8,9) which
include: scimitar syndrome, horseshoe lung, cystic
adenomatoid malformation, and pulmonary
arteriovenous fistula/malformation.
In the scimitar syndrome, the anomalous vein drains
into the inferior vena cava or at its junction at the right
atrium. This vein has the appearance of a scimitar.
This may or may not be accompanied by hypoplasia of
the right lung and dextrocardia, anomalies of the lobes
of the right lung, hypoplasia of the right pulmonary
artery, and an anomalous systemic vascular supply to
the lung (10).
The horseshoe lung is a rare congenital anomaly. It
is associated with some of the findings of the scimitar
syndrome. There is an isthmus of pulmonary tissue
which extends from the right lung base across the
midline behind the pericardium and then fuses with the
left lung base. Likewise, there may be an anomalous
systemic supply (1).
The cystic adenomatoid malformation is an
abnormality of the pulmonary parenchyma due to an
overgrowth of bronchioles (1). There is usually a normal
vascular supply, however there may be an aberrant
systemic artery.
Lastly, the pulmonary arteriovenous
fistula/malformation consists of an abnormal pulmonary
artery and venous connection (1). In this condition,
there is normal pulmonary parenchyma (1) .
Regardless of which variant is present, a diagnosis is
suggested clinically and confirmed with angiography.
Bibliography:
1. Felker RE, Tonkin ILD. Imaging of Pulmonary
Sequestration. AJR. 1990;154:241-249.
2. Nicolette LA, Kosloske AM, Bartow SA, Murphy
S. Intralobar Pulmonary sequestration: a clinical and
pathological spectrum. Journal of Pediatric Surgery
1993;28(6):802-805.
3. Sugio K, Kaneko S, Yokoyama H, Ishida T,
Sugimachi K, Hasuo K. Pulmonary sequestration in
older child and in adults. Int Surg 1992;77:102-107.
4. Javaid A, Aamir AUH. Pulmonary sequestration:
a case report and review. Respiratory Medicine
1994;88:65-66.
5. Lin CH, Lin CT, Chen CY, Peng HC, Chen HC,
Wang PY. Pulmonary sequestration. Chin Med J
(Taipei) 1994;53:168-172.
6. Savic B, Birtel FJ, Knoche R, et al. Pulmonary
Sequestration. In: Frick HP, Harnack GA, Martini GA
et al (eds). Advances in Internal Medicine and
Pediatrics. New York, Springer-Verlag, 1979, pp.
58-92.
7. John PR, Beasley SW, Mayne V. Pulmonary
sequestration and related congenital disorders: A
clinico-radiological review of 41 cases. Pediatr Radiol
1989;20:4-9.
8. Louie HWt Martin SM, Mulder DG. Pulmonary
sequestration: 17-year Experience at UCLA. The
American Surgeon 1993;59:801-805.
9. Clements BS, Warner J. Pulmonary
sequestration and related congenital
bronchopulmonary-vascular malformations:
Nomenclature and classification based on anatomical
and embryological considerations. Thorax
1987;42:401-408.