Patient participation in cancer clinical trials at the University of Hawaiʻi Cancer Center and across the nation began to decline in 2020 due to the pandemic. Clinical centers experienced staffing shortages which limited patients’ access to these trials that offer cutting-edge treatments and provide the highest level of care with outcomes leading to more patients being cured of cancer, living longer and improving their overall quality of life.

With access to 85 different clinical trials, the UH Cancer Center has ramped up efforts to bring patient participation back to pre-pandemic levels through reorganization, hiring new staff and implementing new strategies.
“The decline in patient participation we’ve seen in recent years at the UH Cancer Center reflects what’s going on across the country due to workforce shortage, patient attitudes and the convenience afforded by telehealth. It’s critical to the success of our clinical trials that we resume our activities,” said Jonathan Cho, medical director of the Clinical Trials Office (CTO), which provides infrastructure and operational support for cancer clinical trials at the UH Cancer Center and the Hawaiʻi Cancer Consortium.
The UH Cancer Center remains dedicated to investigating new ways of delivering cancer care to Hawaiʻi’s multi-ethnic population—often underrepresented in clinical trials nationwide. As a result, Hawaiʻi can potentially contribute significantly to expanding the knowledge of how various cancers affect different races, including minority populations.
New changes implemented
CTO team members assist both faculty and industry sponsors throughout the entire lifecycle of a clinical trial, from inception to study closeout. The office serves as a point of contact, coordinating with investigational pharmacy, laboratory, nursing, the National Cancer Institute, investigational sites and other collaborating academic institutions.
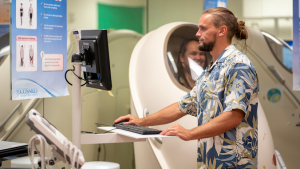
Based on survey results received from clinical trials centers across the nation, CTO has implemented changes including reorganizing workload and hiring additional staff to oversee its various areas. With a new career development program in place, clinical research associates who work closely with trial participants through every step, will be mentored and receive formal training and education from a nurse to advance their skills and grow their career. In addition, their compensation packages have also been improved.
Looking ahead, the UH Cancer Center hopes to expand access to the neighbor islands and increase the number of trials available to Hawaiʻi residents.
“Everyone should consider participating in clinical trials. For patients with a cancer diagnosis, a clinical trial may offer the chance of receiving better treatment for their cancer,” said Cho.
The first phase of construction of the Early Clinical Research Center began in fall 2022 and is tentatively scheduled to be completed in 2024. The center will be the first of its kind in the state, providing access to Phase 1 trials to cancer patients from Hawaiʻi, so they do not have to travel to the continental U.S. for specialized treatments. Phase 1 trials represent cutting-edge cancer treatments and are often considered when patients have a particularly challenging form of cancer or when standard treatments have been unsuccessful.
New publication
The UH Cancer Center dedicated the fall edition of its bi-annual newsletter, Innovations, to cancer clinical trials. The publication provides an overview of the importance of clinical trials, recruitments for local studies and information on how to participate. Read the publication for more.