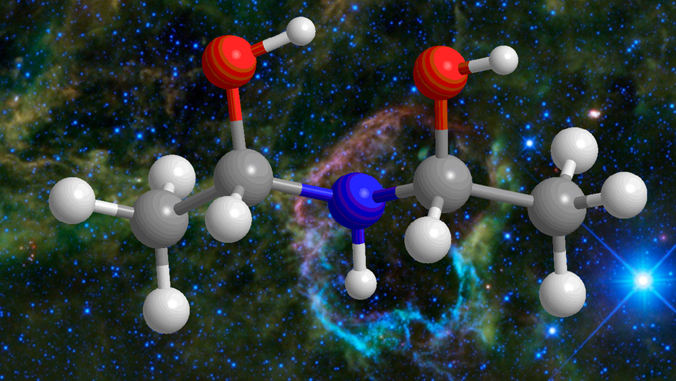
Ices on interstellar nanoparticles and on comets, and the molecules they carry, may have played a much bigger role in starting life on Earth than originally thought, according to University of Hawaiʻi at Mānoa researchers. Molecules assembled from RNA (ribonucleic acid) and proteins are widely considered to be among life’s first building blocks. UH research suggests new routes to RNA replication assisted by molecules expected to be readily formed from ingredients abundant in space.
Using technology in UH Mānoa’s W. M. Keck Research Laboratory in Astrochemistry, the team out of the Department of Chemistry led by Professor Ralf Kaiser recreated the environment found inside dense interstellar molecular clouds. Postdoctoral fellows Joshua Marks and Jia Wang took simple molecules (ammonia and acetaldehyde) and froze them at −441 °F (5 Kelvin) to form a simplified model of interstellar ice. The ice was then slowly heated to 116 °F. This replicated the conditions experienced by ices during the formation of stars, like our sun, in the phase of the gravitational collapse of the molecular cloud. The initial components of the model ice were found to react and produce a variety of larger molecules such as prebiotic (leading to the origins of life) chelating agents detected in the laser laboratory which can form complexes with ions such as sodium, potassium, magnesium, and calcium ions.
“These findings reveal a complex set of reactions that yield prebiotic bonding agents, fundamentally enhancing our knowledge of the level of molecular complexity of organic molecules in deep space,” Kaiser said. “The presence of these molecules enables RNA replication, and once introduced through meteorites and comets onto early Earth they contribute the chemical prerequisites for the origins of life.”
These experiments simulated chemistry that occurs during the formation of a new star. Under these conditions, the simple reactants produced more complex molecules which are predicted to be ideal candidates for assisting in RNA replication when combined with naturally occurring metal ions. Once a new star system is created, the molecules observed here can be introduced to newly formed planets and promote chemistry leading to the origins of life.
The findings were published in Chem in November 2023.